Which Statement Best Describes an Oxidation Reduction Reaction
This is a redox reaction in which octane C8H18 is oxidized. However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons.

Chemistry Reduction And Oxidation Reactions Wikiversity
A Electrochemistry is the study of only oxidation reactions.

. The type of reaction that is shown is. A chemical reaction in which electrons are transferred between reactants. These reactions involving electron transfers are known as oxidation-reduction or redox reactions.
Because of this in many cases H 2 O or a fragment of an H 2 O molecule H or OH in particular can participate in the redox reactionAs such we need to learn how to incorporate the solvent into a balanced redox equation. A chemical reaction in which electrons are transferred between reactants. Which of the following is true about a redox reaction.
O O O If there is a change in the oxidation states of atorns from reactants to products the reaction is an oxidation- reduction reaction If there is formation of salt and water the reaction is an oxidation-reduction reaction. The reaction that takes place in a chemical cell is best classi ed as A. The sharing of a pair of electrons between two atoms a relatively weak bond.
A chemical reaction that involves oxygen C. In this reaction iron is reduced and copper is oxidized In this reaction both iron and copper are oxidized Which statement best describes the following reaction. A chemical reaction in which electrons are transferred between reactants 2Beryllium Be has four electrons.
It permits the migration of ions In what kind of cell are the redox reactions made to occur by an externally applied electrical current. C Iron transfers two electrons to copper. Cl2 2e 2Cl C.
B Iron changes into copper. The correct statement that describes a Redox reaction is D. The attraction between two charged atoms a relatively weak bond in an aqueous solution.
In a redox reaction an electron is lost by the reducing agent. Which of the following statements best describes how a reducing agent in is chemically altered in a biological redox reaction. A The reaction occurs in a voltaic cell and releases energy.
A chemical reaction that involves oxygen. 1Which statement best describes an oxidation-reduction reaction. But when an element is reduced it gains electrons.
Third option is the correct one. The chemical formula that shows the correct subscripts is D BeF₂. In redox reactions a reduced half and an oxidized half occur together.
When an object is electroplated the occurrence of a redox reaction is nonspontaneous and it requires an electric current. A chemical reaction that involves oxygen C. So here reducing agent is a substance a substance that reduces other and oxidized itself.
Which statement best describes how a salt bridge maintains electrical neutrality in the half cells of an electrochemical cell. Which statement best describes the reaction represented by the equation2NaCl 2H2O electricity -- Cl2 H2 2NaOH. C decomposition reaction.
The sharing of a pair of electrons between two atoms a relatively strong bond. B The reaction occurs in a voltaic cell and absorbs energy. Nadh is oxidized by fadh2 which is then immediately oxidized in complex complex ii.
1Which statement best describes an oxidation-reduction reaction. Chemistry 25022021 1840 olivia0420. Which of the following statements best describes an ionic bond.
Which answer best describes what is happening in the following reaction. The presence of which reactant is the best indicator of an oxidation-reduction reaction. Which statement best describes the relationship between electrochemistry and oxidation-reduction reactions.
A chemical reaction in which there are fewer products than reactants B. Fe Cu2 Fe2 Cu A Two electrons are lost. Mg Mg2 2e D.
A chemical reaction in which there are fewer products than reactants. Which equation represents the half-reaction that takes place at. A Electrochemistry is the study of only oxidation reactions.
Which statement best describes what happens during the redox reaction between nadh and complex i of the electron transport chain. This is the basis of redox reactions. Nadh is oxidized as a pair of electrons which are transferred to the etc and 4 four h are pumped into the intermembrane space.
Olt gains a hydrogen atom and loses potential energy O It loses a hydrogen atom and loses potential energy It loses a hydrogen atom and gains potential energy. Behave given christian S here which of the following statements best describes how our reducing agent in is chemically altered in a biologically dogs reaction. 4Al 302 2Al2O3 In this reaction aluminum is oxidized and oxygen is reduced In this reaction aluminum is reduced and oxygen is oxidized In this reaction th aluminum and oxygen are oxidized This is not an.
Chemistry 19092021 2310 NetherisIsTheQueen. A chemical reaction in which there are fewer products than reactants B. When an element is oxidized it loses electrons.
D Copper transfers two electrons to iron. C Oxidation-reduction reactions are the basis of electrochemical cells. C The reaction occurs in an electrolytic cell and releases energy.
A Redox reaction oxidation-reduction reaction involves the exchangetransfer. O lt gains a hydrogen atd and gains potential energy. An oxidation-reduction redox reaction is a type of chemical reaction in which electrons are transferred between chemical species.
So reducing agent is a substance that produces others and oxidize itself. A chemical reaction in which electrons are released from the system D. Which statement best describes the oxidizing and reducing abilities of the reactants.
1Which statement best describes an oxidation-reduction reaction. A chemical reaction in which electrons are released from the system. B Electrochemistry is the study of only reduction reactions.
If there is formation of a precipitate the reaction is an oxidation-reduction reaction. B Electrochemistry is the study of only reduction reactions. E Two electrons are gained.
A chemical reaction in which electrons are released from the system D. So here first we will discuss that What is reducing agent. Which statement best describes what changes occur over the course of the following oxidation-reduction reaction.
In the reaction MgCl2MgCl2 the correct half-reaction for the oxidation that occurs is A. Which statement best describes the relationship between electrochemistry and oxidation-reduction reactions. Silver ion Ag is a stronger oxidizing agent than copper ion Cu2 and copper metal is a stronger reducing agent than silver.

Oxidation And Reduction In Organic Chemistry Master Organic Chemistry
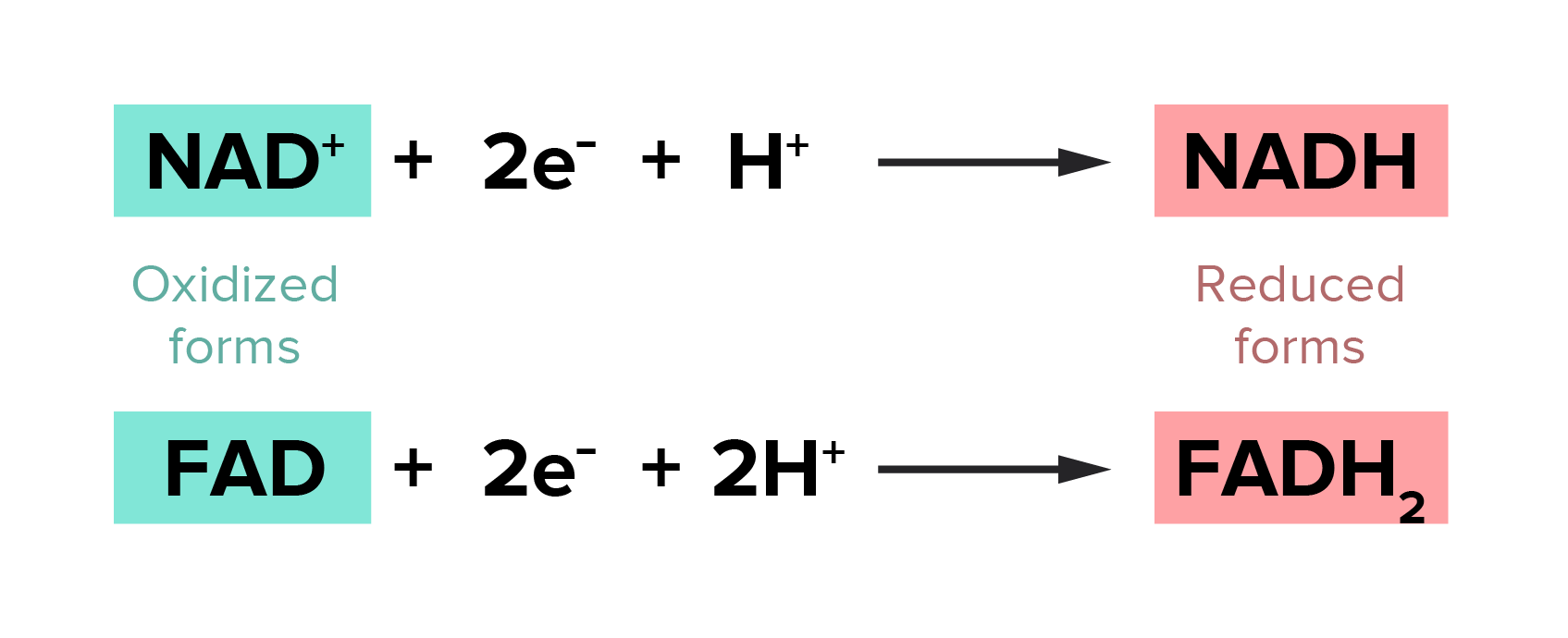
Oxidation And Reduction Reactions For The Mcat Everything You Need To Know Shemmassian Academic Consulting
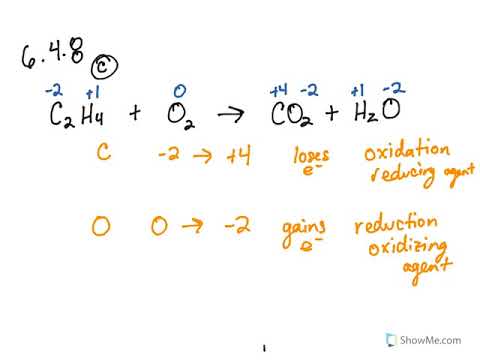
6 5 Classifying Chemical Reactions Redox Problems Chemistry Libretexts
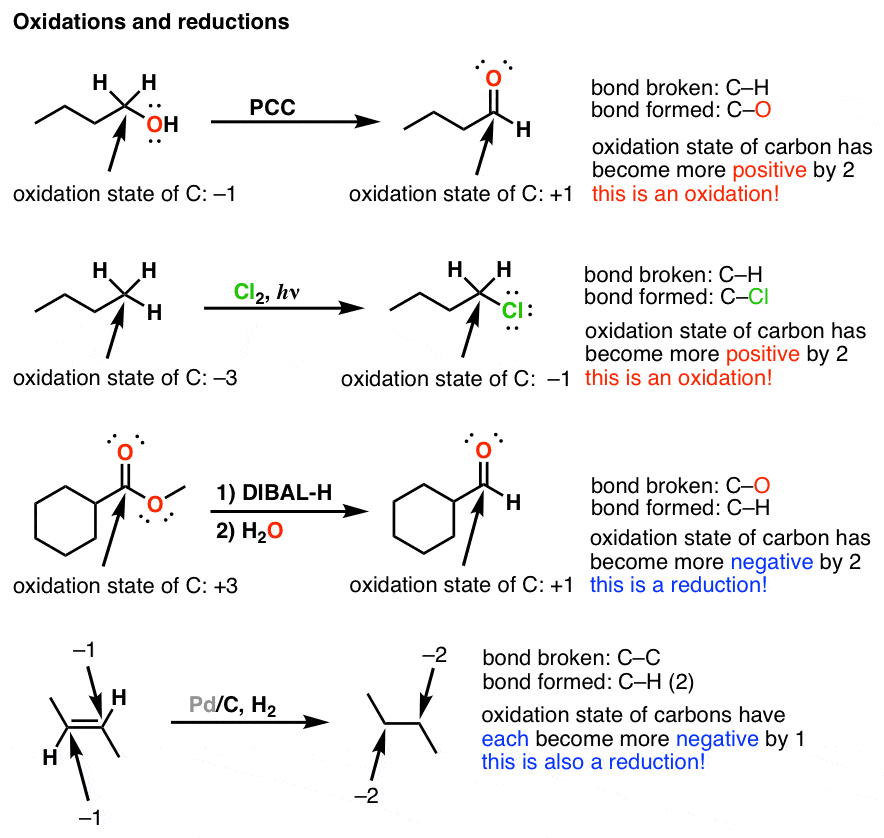
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry

Oxidation Reduction Reactions Boundless Chemistry

Chemistry Notes Teaching Science Chemistry Class
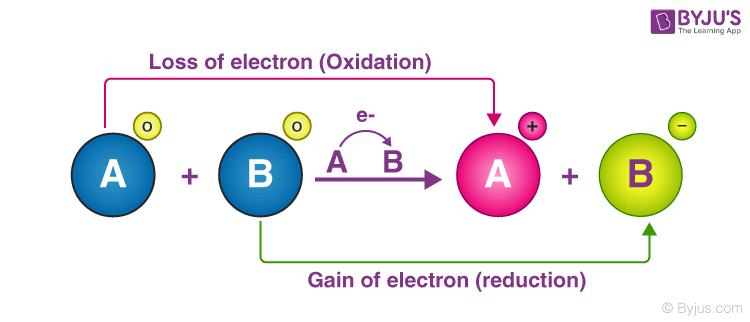
Is Cellular Respiration An Oxidation Or Reduction Reaction
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry

Oxidation Reduction Reactions Boundless Chemistry

Balancing A Redox Equation In Acidic Solution Worked Example Video Khan Academy

Electrochemical Series Definition Chart Applications Reduction Potential Redox Reactions Reducing Agent

Catalysts For Fine Chemicals Synthesis Regio And Stereo Controlled Oxidations And Reductions Volume 5 Series 5 Hardcover Walmart Com In 2022 Hardcover Synthesis Chemical
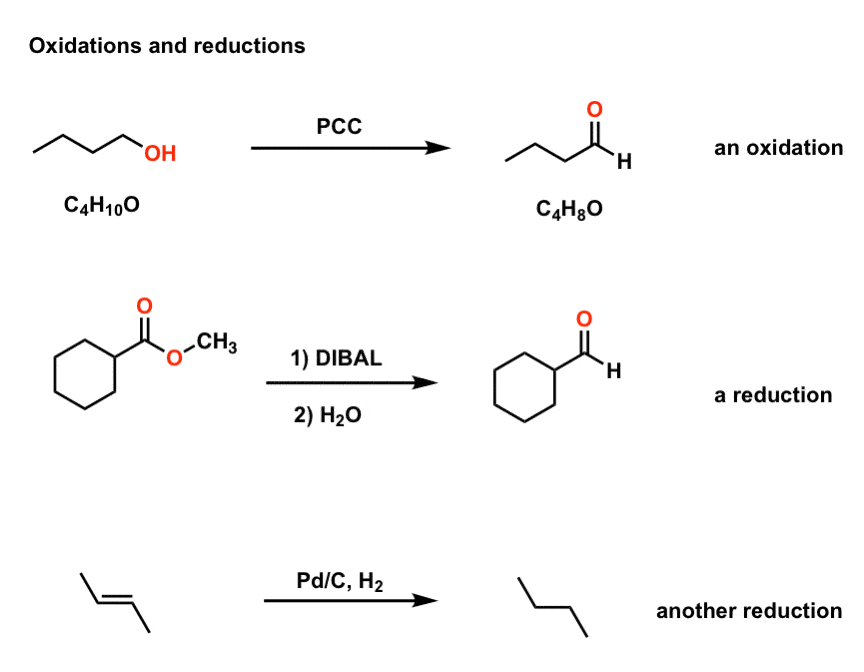
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry

Redox Reactions Biology For Majors I

Oxidation Reduction An Overview Sciencedirect Topics

Vitamin Water Label Template Elegant Vitamin Water Label Nutrition Facts Nutrition Facts Bla Label Templates Bottle Label Template Water Bottle Labels Template
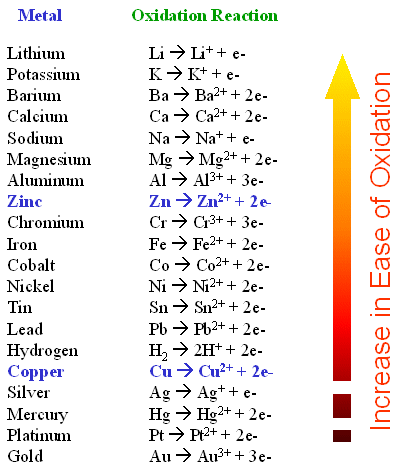
Other Oxidation Reduction Reactions

Electrophilic Aromatic Substitution Mechanism Master Organic Chemistry Organic Chemistry Organic Chemistry Books Organic Chemistry Study
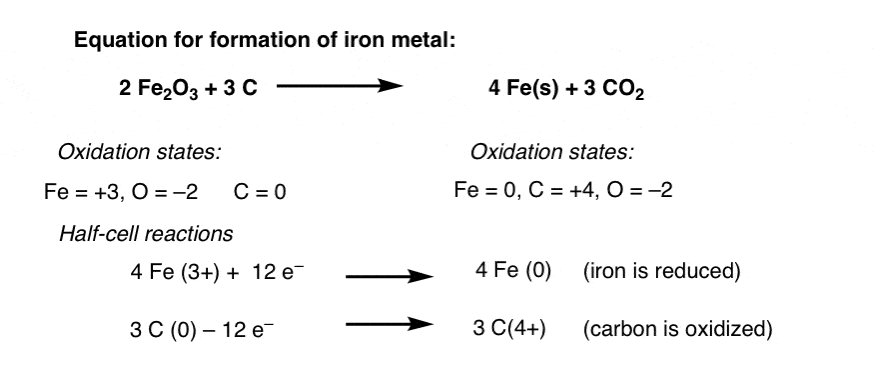
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry
Comments
Post a Comment